The Baltic Healthtech Map 2024 aims to thoroughly explore the growing healthtech market and innovative companies in the Baltic region. Our research gathers data on various projects, technologies, startups, and key stakeholders focused on improving quality of life, healthcare access, medical tourism, longevity, and fostering a supportive ecosystem for healthtech startups. The result will be the Baltic Healthtech Map 2024 — a unified resource for everyone in the ecosystem.

We are surveying founders of innovative companies, innovation support representatives, service providers, development and startup support institutions, and venture investors.
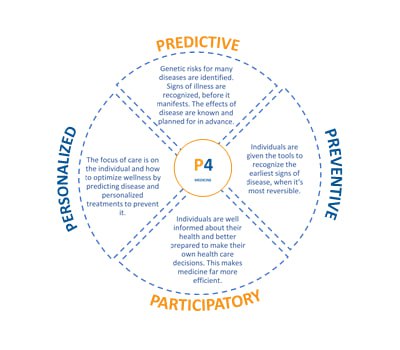
OBJECTIVES OF THE RESEARCH:
– Introduce stakeholders and professionals in healthcare, health preservation, and life quality sectors to innovative technologies being developed or already in use in the Baltics.
– Showcase a range of solutions addressing modern healthcare challenges.
– Create a comprehensive directory of services and infrastructure available to innovative healthcare companies in the Baltics, promoting future collaborations and joint projects.
– Identify growth opportunities and potential areas for national specialization in the global market. For example, we see great potential for developing medical tourism in the Baltics, which will require collaboration among diverse stakeholders and advanced healthcare technologies.
CATEGORIZATION OF COMPANIES:
Our research methodology is rooted in a comprehensive analysis of innovative companies within Baltics and a study of global trends in the Healthtech market.
To provide a multifaceted view of the modern healthcare innovation ecosystem, we have categorized technologies into 11 sections:
Technologies and solutions grouped by users:
– patients,
– doctors,
– clinics and hospitals,
– pharmacies (Pharma Supply Chain),
– pharmaceutical companies
– insurance companies,
– others
Description (main technologies involved)
For doctors: Assisted Prescribing, Clinical Decision Support, Computer-Assisted Physician Documentation
For pharmacies (Pharma Supply Chain): Asset Tracking, Autonomous Pharmacy, Blockchain for Shipping, Cold Chain Packaging, Digital Pharmacy, Inventory Management Software, Last-mile Delivery, Medication Adherence, Price Transparency, Robotic Fulfillment, Visibility Platforms
On the processes of providing medical services
Technologies and solutions grouped by the type of service:
– Disease prevention and Health Promotion,
– Lab Diagnostics & Screening,
– Hospital treatment,
– Home treatment,
– Rehabilitation,
– Home care
Description (main technologies involved)
Lab Diagnostics & Screening: Genetic diagnostics, Point-Of-Care Diagnostics
Home care: Full-stack Home Care, Telehealth, Homecare workflow, Dialysis & Infusion Therapy, Care Management Platforms, Medication & DME Fulfillment, Remote Patient Monitoring, Virtual Care, In-home Diagnostics, Home Care Management Platforms, Patient Directed Scheduling & Navigation, Care Coordination & Care Management
Rehabilitation: Post-Stroke & Physical Rehabilitation, Rehab and Mobility-Assist Robotics
This section covers technologies for drug invention, dietary supplements, supporting development processes, digitalization of processes for pharmaceutical companies and pharmacies
– Drug discovery
– Drug-delivery
– Digital pharma
– Genetics
– Real World Data (RWD)
– Real World Evidence (RWE)
– Rare disease, orphan disease
– 3d-bioprinting
– Other
Description (main technologies involved)
Drug discovery: 3D Bioprinting, Organoids& Organ-on-a-chip, AI-Powered Discovery, Single-Cell Analysis, Bio-Simulation, Protein Discovery & Design, Synthetic Biology
Drug-delivery
Digital pharma
Genetics: Gene Editing, Genetic Screening & Testing, Molecular Diagnostics, Genomic Profiling
Real World Data (RWD)
Real World Evidence (RWE)
Rare disease, orphan disease: Rare diseases are those with an incidence of less than one in every 2,000 people in Europe. Orphan diseases are poorly researched conditions, diseases for which specific treatments are not yet known, and diseases of limited interest to scientists and doctors. Patients with such diseases often feel neglected and “lost” in the world of health care.
3d-bioprinting
Other
These markets highlight various analytical resources, such as Sifted, CB Insight and others, to attract the attention of investors.
Description (main technologies involved):
AgeTech/SilverTech – Solutions for people 60+
FemTech and Men’s health – Women’s health technology: Fertility (incl. Screening & diagnostics and Tracking & monitoring), Gynecological health, Menstruation, Menopause, Sexual health & wellness (incl. Contraceptives), Maternal & fetal health. Technology for men’s health
EduMedTech – educational technologies for healthcare
Techbio – The focus is on the ‘technology’ aspect of biotechnology – think of start-ups developing platforms and infrastructure to improve drug discovery, create new food ingredients or reduce our impact on the climate.
Dentaltech – Transforming dental care. Dental prevention and treatment technologies
Mental health – Mental health
Rehabtech – Technology for rehabilitation
Digital health – Digital Health
Digital Therapeutics – Digital therapy
Telehealth (telemedicine) – Health Care Delivery Using Telecommunications Technology: Telemedicine Providers, Platforms & Marketplaces (incl. Hybrid In-Person/Virtual Care), Telepharmacy, Teletherapy, Coaching & Care Management (incl. Direct-to-Consumer), Virtual/Digital Care Enablement, Remote Monitoring, Screening and Diagnostics, Teletherapy, Wellness Coaching and Chronic Care Management, Telehealth Software and Connectivity Solutions
Clinical Trials Tech – Clinical Trials Technology
Foodtech – Healthy Nutrition
SportTech / Wellness – Technology for an Active Lifestyle
– Oncology
– Diabetes (Type 1 & 2)
– Orphan disease
– Behavioral health (incl. Anxiety & Depression, Sleep)
– Injuries
– Obesity
– Substance Abuse and Narcology, Smoking Cessation
– Chronic Pain
– Оther
– Brain disease / Neuroscience
– Ophthalmology
– Heart diseases / Cardiology
– Digestive / Gastroenterology
– Lung diseases
– Kidneys and liver
– Musculoskeletal & Orthopedics
– Nervous system
– Genitourinary system
– Other
Description (main technologies involved)
Brain – Global incidence and prevalence of neurological disorders such as dementia, epilepsy, Parkinson’s disease, Huntington’s disease, Alzheimer’s disease, headaches, migraine, multiple sclerosis, neuroinfections, stroke and cerebral palsy, mental health (Anxiety & depression, Sleep, schizophrenia, etc.)
– Idea, prototype, experimental proof of concept (TRL 1-3)
– Laboratory sample (TRL 4)
– Technology in relevant environment. Preclinical tests (TRL 5)
– Technology demonstrated in relevant environment. Phase 1 clinical trials (TRL 6)
– Certification and Pilot Phase (TRL 6)
– System prototype demonstration in operational environment. Phase 2 clinical trials (TRL 7)
– System complete and qualified. Certificate availability (CE mark). Sales validated (TRL 8-9)
– Other. The level of readiness is uncertain
– Sensors and wearable devices
– Equipment
Description (main technologies involved)
Section includes wearables and equipment for:
– Remote Patient Monitoring
– Point-of-care Testing
– Virtual Reality Diagnostics & Therapeutics
– 3D and Bioprinted Devices
– Post-stroke & Physical Rehabilitation
– Smartphone-based Diagnostics & Therapeutics
– Wearables & Sensors
– Surgical Intelligence
– Computer-aided Diagnostic Imaging
– Glucose Monitoring Technology (Noninvasive Glucose Detection)
– Wireless Connectivity
– Bioelectronic Neuromodulation
– Robotic and Image-guided Surgery
– Rehab and Mobility-Assist Robotics
– AI/ML for Healthcare
– AR/VR
– Medical Information Systems (MIS)
– IT infrastructure
– Cybersecurity
– Hospital care
– Clinical workflow
– Digital biomarkers
– Clinical Decision Support
– Digital Twins & Biosimulations
– Other
Description (main technologies involved)
AI/ML for Healthcare
– Admin & Operations (incl. Digital Front Door, RPA, Underwriting & Claims, Revenue Cycle Management, Patient Engagement (Payers), EHR Management and Virtual Scribes)
– Monitoring & Diagnostics (incl. Radiology AI, Pathology AI, Remote Patient Monitoring, Point-of-Care (in clinic))
– Tech Infrastructure (incl. DataOps & Data Science Platforms)
– CareDelivery (incl. Specialty Care, Surgical Tech, Chronic Disease Management, ICU Tech, Primary Care)
– Drug Development & Research (incl. Drug R&D, Medical Data Repositories, Clinical Trial Tech)
– Medical Imaging AI
IT infrastructure
– Quantum Computing
– Cloud R&D Software
Cybersecurity
– Secure Clinical Communication
– Endpoint Security
– Threat Intelligence
– Managed Security
– Third-Party Risk
– Network Security
– SIEM
– Secure Collaboration
– Data Security
– Website Security
– Vulnerability Management
– Cloud Security
– Human Element
– Data Privacy (HIPAA)
– Medical Device Security
Hospital care
– Data&information management
– Diagnostics (Pathology & Radiology)
– Patient intake & virtual waiting rooms
– Referral management
– Revenue cycle management
– Scheduling
– Self-trage & navigation
– Surgical intelligence
– Patient flow
– Patient monitoring
– Patient Data & Analytics
Clinical workflow
– Electronic Health Record Augmentation
– Patient Flow & Command Center
– Provider Staffing & Human Capital Management
– Referral Management
– Pre-Visit Patient Management
– Virtual Assistants
– Provider Search & Scheduling
– Patient Engagement
– Population Health Analytics
– Risk Adjustment Technology
– Utilization Management
– VBC Contract Management
– Clinical Exam Tools
– Self-Triage & Navigation
– Cost Reduction (incl. Care Management & Coordination, Managed Care, Utilization Management, Value-Driven Providers, Remote Patient Monitoring)
– Administartive Tools (incl. Populations Health Analytics, CQM Capture, SDOH Screening & Refferal Management, Contract Management, Risk Adjustment Coding Capture)
Digital biomarkers
– Vital signs
– Sleep
– Mobility and activity
– Visual
– Auditory
– Data analytics & clinical trials platforms
– Sweat & saliva
– Urine & feces
– Blood testing (incl. Blood glucose)
– Innovation recipient/implementator (companies interested in collaboration with startups)
– Services (IP registration, certification, contract manufacturing, IT)
– Resources (accelerators, Venture builder, VC, grantors….)
– Solutions piloting sites (clinics, insurance companies,….)
– Startup Development and Support Institutions
– (Associations, clusters, government agencies, media)
– Institutes, laboratories, research centers (training + research)
– Regulator
– Other
As the healthcare ecosystem evolves, the map will be updated. We are committed to improving the map’s structure and detail, especially where multiple companies operate in the same field. If you think a category is missing or want to suggest a new one, please contact us at hello@4pmventures.com, and we will consider your input.
CRITERIA FOR INCLUSION ON THE MAP:
– Companies primarily focused on healthcare technology, serving stakeholders (suppliers, patients, regulators), or working in healthcare and life sciences (pharmaceuticals, medical devices), or offering services/collaborations with development-focused companies.
– A physical presence in the Baltics (mandatory for startups), through a headquarters, representative office, or branch office.
– Providing relevant information, including a website, contact person, contact details, a brief self-description, and a logo.
– A team with at least two members.
If you believe your company should be included on the map or if you want to update the information displayed, please fill out the form provided.
We hope the Baltic Healthtech Map 2024 will provide a comprehensive view of startups and the scope of medical technology development in the Baltics, showcasing the region’s readiness and potential to attract innovative enterprises from around the world.
Thank you for collaborating with us in this important effort!

SmartOmica (Latvia, Riga)
We design, build, and deliver comprehensive methodology to personalize each patients’ profile through in-depth AI-based data analytics and translate the results to clinically applicable language in order to make it a practical tool for dedicated medical practitioners.
Year of foundation: 2020

SkinPort (Latvia, Jūrmala)
SkinPort microneedle array is a painless alternative to hypodermic steel needles that are being used for injecting drugs. SkinPort microneedles operate in the upper layers of skin while not reaching blood vessels and nerves, which makes injections with Skinport painless. Skinport Microneedles have the potential to become a highly progressive device for both drug delivery and monitoring purposes as they penetrate the skin, allowing the delivery of drugs in the upper skin layers and the extraction of body fluids.
Year of foundation: 2021

SmartClast (Latvia, Riga)
The “SmartClast” is a startup that develops an innovative technology of personalized bone grafts. SmartClast technology makes the rate of bioresorption of the bone graft adjustable in accordance with the individual characteristics of each patient.
Year of foundation: 2021

DoctoWell (Latvia, Jūrmala)
A fully integrated platform for clinic operational efficiency improvements and patient-specialist communication.
Year of foundation: 2020

CastPrint (Latvia, Riga)
CastPrint is the missing link for hospitals to introduce 3D printing technology in their daily operations. By reducing patient treatment time by 1/3 and saving more than 15% in labour hours on each patient, CastPrint allows hospitals to offer patients with cutting-edge treatment of fracture trauma. Unlike traditional plaster casts which are heavy, itchy and unhygienic, CastPrint’s 3D printed casts are light weight, waterproof and comfortable to wear, enabling patients to enjoy an active and fulfilling recovery from their injuries.
Year of foundation: 2016

Assistentis (Latvia, Valmiera)
Implementing new technology based solutions in Healthcare organisations.
Year of foundation: 2013

Longenesis (Latvia, Riga)
Longenesis makes data discovery and patient engagement easier for biomedical institutions, patient organisations and research sponsors. Our products empower hospitals, doctors, and researchers to communicate directly while providing compliant and consent-enabled biomedical data curation and generation.
Year of foundation: 2019

Sepsiscan (Latvia, Riga)
Technology and hyperspectral imaging solution for early sepsis diagnostics.
Year of foundation: 2023

Bdetect (Latvia, Riga)
A handheld, point-and-click device for general practitioners to quickly assess skin lesions for melanoma risk.
Year of foundation: 2021
Correcty (Latvia, Riga)
The idea of creating a T-shirt that helps to improve the posture arose naturally as both authors faced this problem and first created this product for themselves.
Year of foundation: 2018

Medon (Latvia, Jūrmala)
Medon is a digital clinic that offers medical consultations online. Stop googling your symptoms, sign up for a visit instead.
Year of foundation: 2020

UPOlife (Latvia, Riga)
We are focusing on timely Atrial Fibrillation and 20 more arrhythmia diagnosis and condition management.
Year of foundation: 2019

Sintez Company (Latvia, Riga)
Application of preventive medicine and food to prevent diseases.
Year of foundation: 2021

Finamba (Latvia, Riga)
We are giving patients ability to easily split their payments for medical procedures with the best offer from various banks
Year of foundation: 2021

BirgerMind (Latvia, Liepaja)
BirgerMind® provides non-invasive Brain-Computer Interfaces (BCIs) that allow completely paralyzed patients to communicate independently. BirgerMind® was created with the goal of helping patients with Amyotrophic Lateral Sclerosis (ALS) and Motor Neuron Disease (MND) regain the ability to use email, social networks, generate voice from text and ask for help, manage contacts and keep track of writings and notes, all using the flexible headgear with ALS-compatible brain signal reading. The main focus is ease of use and quick setup.
Year of foundation: 2021

VREACH (Latvia, Riga)
The VREACH mission is to use the opportunities provided by virtual reality to make the work of functional specialists effective, allowing them to save both material and emotional resources. VREACH also wants to promote public awareness of the rehabilitation process of cognitive abilities, making the sessions and processes transparent to the patient’s child’s family. VREACH sees rehabilitation as an innovative, technology-based modern process that serves faster patient progress, involves the patient’s family in the rehabilitation process, as well as helps to facilitate the work of functional specialists.
Year of foundation: 2020

Skin Bliss (Latvia, Riga)
Skin Bliss is an app that helps you find your perfect skincare match. It is like a Tinder match, but based on science and optimized for lasting relationships.
Year of foundation: 2021

Vigo Health (Latvia, Riga)
Vigo is a digital rehabilitation program for stroke survivors, available on a tablet – in the comfort of your own home and suitable for users without technical training.
Year of foundation: 2018

SpirulinaNord (Latvia, Riga)
Spirulina is a unique microalgae that is one of the most nutritious foods on the planet.
https://spirulinanord.mozello.lv
Year of foundation: 2019

Nutrameg (Latvia, Riga)
Nutrameg is a healthtech startup developing patented nutrition technology for weight management and fitness used by millions of people worldwide.
Year of foundation: 2022

Uppo (Latvia, Pinki)
We created “Uppo” – an ergonomic wrist rest to support those who spend long hours at the computer and don’t want to be interrupted by wrist pain, finger numbness, weakness and other health disorders that lead to Repetitive Strain Injury (RSI) like Carpal Tunnel Syndrome (CTS).
Year of foundation: 2017

Lightspace Labs (Latvia, Mārupe)
Keep your eyes on the patient – AR headset can overlay photorealistic, real-time patient data, enhancing your ability to diagnose, treat, and perform surgery more precisely than ever before.
Year of foundation: 2014

Xoresearch (Latvia, Riga)
XOresearch is a company focused on providing deep learning technology to real-life products for natural language processing and healthcare. XOresearch’s AI lab is a research unit that both offers outsourcing services and develops technologies for company’s products.
https://xoresearch.com/#/xoresearch
Year of foundation: 2015

4PM Ventures – Baltic Healthtech Venture Builder (Latvia, Riga)
We focus on developing and introducing new technologies to the Healthtech market, building businesses that shape the future of healthcare. Our goal is to positively impact human health, enhance quality of life, and expand the reach of medicine, making it accessible to everyone. We’re seeking teams with strong tech skills to co-create and scale companies that are globally focused, working together to change and improve the healthcare system.
Year of foundation: 2019

Latvian Business Angel Network (LatBAN) (Latvia, Riga)
Network of angel investors of Latvia who make investments in startups with exceptional growth potential

Investment and Development Agency of Latvia (LIAA) (Latvia, Riga)
Attracting investment, Promoting foreign trade, Network of LIAA representative offices, Tech transfer, Supporting startups and innovation

Latvian Private Equity and Venture Capital Association (LVCA) (Latvia, Riga)
Our activity is Inform entrepreneurs and the public about the possibilities of receiving venture capital financing; Promote the exchange of knowledge and experience of the members of the association; Represent the interests of the industry to state institutions and legislators;
Organize and ensure cooperation with international and foreign Venture Capital associations.

Latvian Startup Association “Startin.LV” (Latvia, Riga)
We support Latvian startups’ growth by educating, inspiring, and mobilizing the tech community to build the strong economy of tomorrow.

Ministry of Economics (Ekonomikas ministrija) (Latvia, Riga)
The Ministry of Economics coordinates and supervises the systems of national standardization, accreditation, metrology and market supervision. Ministry also plans and manages the provision of measures related to prevention of energy crises. The Ministry of Economics performs the administrative supervision of prospection, exploration and production of hydrocarbons, as well coordinates the medium and long term forecasting of changes in labor market.
Also the function of the Ministry is to introduce and supervise the programs and projects of EU structural funds and other foreign financial means.

Riga Technical University (Rīgas Tehniskā universitāte) (Latvia, Riga)
A modern, internationally renowned and prestigious multidisciplinary technical university – the only one in Latvia

FitRadar (Latvia, Riga)
FitRadar is a new free app to find the best personal trainer or sport event in your local area, in any sport. Free membership. Ad-free. Coming soon.
Year of foundation: 2017

BrunoMaster (Latvia, Kuldīga)
Remote psychoemotional therapy solution. Promoting mental health and wellbeing.
Year of foundation: 2021
Fermentful (Latvia, Mārupe)
Plant-based drinks to support your mental and physical wellbeing.
Year of foundation: 2020

Cellbox Labs (Latvia, Riga)
Developer of miniature organ replicas designed to provide services from concept generation to small-batch fabrication. The company’s organ replicas are focused on microphysiological systems, also known as organs on chip and cancer diagnostics and are manufactured using materials and technologies that combine both the functional specifications and volume scalability for manufacturing, enabling pharmaceutical companies to perform preclinical tests in human-like organs.
Year of foundation: 2020

Anatomy Next (Latvia, Riga)
Information designed to make anatomy easy to remember with 3D models, articles, audio, quizzes, and more
Year of foundation: 2015

SpirulinaNord (Latvia, Latvija)
Spirulina is a unique microalgae that is one of the most nutritious foods on the planet.
Our delicious and healthy spirulina drinks provide a long-term boost to your well-being and energy. No stimulants or caffeine.
https://spirulinanord.mozello.lv
Year of foundation: 2019

Caliverse (Latvia, Latvia)
Meet Caliverse – one of the best calisthenics fitness apps out there with workouts made by professionals. Calisthenics for the whole universe – this is our mission and this is Caliverse. Giving a chance for everyone to learn what is calisthenics and how to perform it correctly – our friends United Calisthenics Group will show exactly how everything is done!
Year of foundation: 2019

AIMUNO (Latvia, Riga)
AIMUNO is a biotech startup that aims to develop an integrative biomarker to distinguish patients who respond to immunotherapy with checkpoint inhibitors from patients with stage III and IV non-small cell lung cancer (NSCLC).
Year of foundation: 2020

VeritaCell (Latvia, Riga)
First to make lab-grade skin cell yields accessible in the operating theatre accelerating and enhancing post-surgical skin renewal with the simplest point-of-care medical kit for surgeons
Year of foundation: 2018

Algae Tree (Latvia, Riga)
Algae Tree is a microalgae-based indoor air quality improvement device that absorbs CO2 from the room, releases oxygen, and manages humidity. Designed for offices with insufficient air quality, it improves human decision-making skills, well-being, and health
Year of foundation: 2022

CO2 Maple (Latvia)
Our task is to remind people about room ventilation and to ensure that people pay attention to air quality.

BrunoMaster (Latvia, Jūrmala)
READING plays a vital role in promoting a person’s intellectual and emotional development, as it stimulates imagination, expands vocabulary, improves the ability to concentrate, see and distinguish the important from the unimportant, expands the perception of the world, and provides satisfaction and joy. Brunomaster’s reading and learning solutions help students achieve their goals in education and realize their potential in life.
Year of foundation: 2016

PrintyMed (Latvia, Riga)
PrintyMed is a revolutionary medicine company that focuses on the development of artificial spider silk for different medical applications.
Year of foundation: 2023

Fitodenta (Lithuania, Vilnius)
FITODENTA IS THE INNOVATIVE ALTERNATIVE IN POST-OPERATIVE ORAL CARE. Fitodenta natural products promote healing, help to alleviate inflammation and speed up post-surgery recovery times up to 40%. Clinically tested & proven.

INNOSENSUS (Lithuania, Vilnius)
It is estimated that 1 of 3 Europeans do not tolerate gluten. Same in USA. These gluten intolerant people are avoiding to eat in the public places: restaurants, cafes, schools, even kindergartens, avoid traveling. So they are always stress if they need to eat outside the home. Problem: There is no quick and effective way to detect the presence of gluten in the food. Therefore people who are gluten intolerant day by day meets with unpleasant symptoms and this causes physical and psychological problems. SOLUTION: We are creating a personal biosensor, which quickly detects the presence of gluten in the food, and helps to prevent unpleasant symptoms.
Year of foundation: 2021

Vow Health (Lithuania, Vilnius)
The VOW platform is a simplified and fast way to check your health and prevent future health problems. The user’s blood sample is taken and tested in one of the partner laboratories, and the results of the tests are evaluated and commented on by a VOW Health doctor. The user can also track changes in their health from data stored securely on their profile.
Year of foundation: 2020

Viveo Health (Estonia, Tallin)
Future-proofing health insurance businesses. Viveo provides a white-label platform for setting up your world-class customer experience in health & wellness services.
Year of foundation: 2020

Upcoming Health (Lithuania, Vilnius)
Your healthcare can be better. We believe that seeing a doctor should not take weeks. Nor should you spend hours getting there or guessing what are your next steps after the visit.
We’re building the digital well-being platform for today. A place where taking care of yourself and your loved ones becomes a lifestyle, not a burden.
Year of foundation: 2021

Pulsetto (Lithuania, Vilnius)
Pulsetto is a new, world-leading technology that stimulates the vagus nerve – the major highway between your brain and internal organs. Stimulating the vagus nerve increases its activity, resulting in a drop in heart rate, an increase in HRV (heart rate variability), and the activation of the parasympathetic nervous system. This makes you calmer, less stressed, less anxious and results in better sleep.
Year of foundation: 2021

DiaWiser: Your next-gen diabetes care (Lithuania)
DiaWiser is revolutionizing diabetes care with our next-generation AI agent. Designed to be your trusted companion in managing diabetes, DiaWiser offers personalized support, medical guidance, and lifestyle choices tailored to your unique needs and goals.

Wosome (Lithuania, Vilnius)
Personalised supplements for women based on their menstrual cycle specifics. Designed for womenwho care about their health and want to solve problems related to hormonal imbalance.
Year of foundation: 2023

Ligence (Lithuania, Vilnius)
Cardiac ultrasound is a safe and inexpensive test for monitoring and preventing heart disease. Manual work (e.g. measuring, writing report) takes 50-85% of test time. We make cardiac ultrasound more accessible, cheaper and less human reliant by automating the analysis.
Year of foundation: 2019

Oxipit (Lithuania, Vilnius)
Oxipit is a computer vision software startup specialized in medical imaging. With a team of award-winning data scientists and medical doctors, the company aims to introduce innovative Artificial Intelligence/Deep Learning breakthroughs to everyday clinical practice. Oxipit is the authors of CE certified multi-award winning ChestEye radiology imaging suite.
Year of foundation: 2017

Sentante (Lithuania, Vilnius)
Longenesis makes data discovery and patient engagement easier for biomedical institutions, patient organisations and research sponsors. Our products empower hospitals, doctors, and researchers to communicate directly while providing compliant and consent-enabled biomedical data curation and generation.
Year of foundation: 2017

Biomatter (Lithuania, Vilnius)
Creating proteins and enzymes with Intelligent Architecture™ for health and sustainable manufacturing.
Year of foundation: 2023

Allergomedica (Lithuania, Vilnius)
Allergomedica is a digital first allergy clinic. Our aim is to help people fully understand their allergies and find an efficient ways to improve their life quality by leveraging modern technology.
Allergomedica offers allergy diagnostic programs based on molecular allergy blood test. It provides a detailed explanation of yours allergy profile and set of recommendation and treatment options.
Year of foundation: 2016
Rīga Stradiņš University (Latvia, Riga)
One of the most modern universities in the Baltic States with an extensive choice of study programmes in health care and social sciences, a strong foundation in research and international recognition.

Ministry of Health (Veselības ministrija) (Latvia, Riga)
The Ministry of Health is the leading public authority in the health sector. It is responsible for public health, healthcare and pharmaceuticals. The main task of the Ministry is to develop and implement public policies to ensure public health in a healthy environment, by promoting prevention, promoting healthy lifestyles and creating the conditions for the population to receive cost-effective, accessible and quality healthcare services.

Migrevention (Estonia, Tartu)
Migrevention Digital Headache Clinic provides a holistic, evidence-based approach to managing primary headaches. We equip both patients and specialists with simple yet powerful digital tools, enabling more accessible, collaborative and 10x more efficient disease management pathway through the thoughtful use of most relevant data

Certific (Estonia, Tallin)
Reduce admin. Elevate care
With a single, ready-to-use platform, you can communicate with patients, receive automated medical summaries, and manage tasks.
Year of foundation: 2020

MX Labs (Estonia, Tallin)
Health Monitoring with Face‑Scanning Technology.
Clinically validated software for vital‑signs measurements.
Year of foundation: 2020


Nanordica Medical (Estonia, Tallin)
ADVANCED ANTIBACTERIAL WOUND CARE PRODUCTS
/div>

Antegenes (Estonia, Tartu)
Antegenes is a healthcare technology company developing and implementing innovative genetic tests based on polygenic risk score technology to enable personalised prevention of common cancers.
Year of foundation: 2018

HEALTH FOUNDERS (Estonia, Tallin)
We are a launchpad for ambitious founders who are building the future of health

Diagnolita (Lithuania, Vilnius)
A start-up company developing innovative molecular diagnostics assays. We aim to translate achievements of genomics and our deep knowledge as well as experience in life science technologies such as qPCR, NGS, etc. to molecular diagnostics for medicine.
Year of foundation: 2013

Delta biosciences (Lithuania, Vilnius)
We are on a mission to accelerate early stage drug discovery by miniaturising functional assays and exponentialising the chemical space.
https://www.deltabiosciences.com/
Year of foundation: 2020

Atrandi Biosciences (Lithuania, Vilnius)
This biotech startup, based in Vilnius and Denver, offers an arsenal of high-throughput droplet microfluidic and next-gen single-cell analysis tools, which are used in life science lab experiments and research. Its proprietary semi-permeable capsule technology is opening new application opportunities across multiple life sciences domains.
Year of foundation: 2016

Mindletic (Lithuania, Vilnius)
Mindletic is a digital mental health gym, co-created with certified psychologists and mental health organizations, aiming to predict & prevent emotional imbalance and increase emotional intelligence (EQ), a key human competency that can’t be replaced by AI in the future
Year of foundation: 2020

Biotecus (Lithuania, Vilnius)
We are working with high quality hemp biomass from Lithuania in order to create potent and compliant products.
Year of foundation: 2017

Health Tech Accelerator (Lithuania, Vilnius)
The HealthTech Accelerator is a community of fearless innovators enabling one another to thrive in the tech world. We create and drive an ecosystem for visionary entrepreneurs who are shaping the future of healthcare. This initiative aims to bring together stakeholders and contribute to the growth of a robust health tech ecosystem by nurturing talent, promoting research and development, and advocating for a culture of innovation. The project is initiated by the Future Leadership Innovation Agency, along with a wide range of private and public partners, including agencies, pharmacists, hospitals, and individual enthusiasts.

Kilo Health (Lithuania, Vilnius)
Dynamic company in digital health, with driven entrepreneurs. Thriving startup studio with 30+ successful products.
Cardio.AI (Latvia, Riga)
XOresearch’s Artificial Intelligence for Automatic Annotation and Interpretation of Electrocardiogramsand recorded by any legally marketed source. This software medical device enables unattended cardiac monitoring with a seamless EHR interface.
https://cardio.ai/#/whycardioai
Year of foundation: 2015
